
Medical device industry has been escalating since 2020 because people have aware of their clinical conditions, particularly after Covid-19 pandemic, the market causes expansion of new medical device manufacturers in 2021. Apart from a medical device production, laboratory testing at critical phases is also evitable in the business in which both physical-chemical and biological tests have to be carried out. The use of laboratory animals to evaluate the efficacy or toxicity of medical devices is thus of tremendous importance. The standard that applied to testing is ISO10993 of International Standard Organization which consists of multiple test guidelines such as cytotoxicity (ISO10993-5), sensitization (ISO10993-10), and irritation (ISO10993-23).
By Chanika PINYOROSPATHUM, Ph. D.
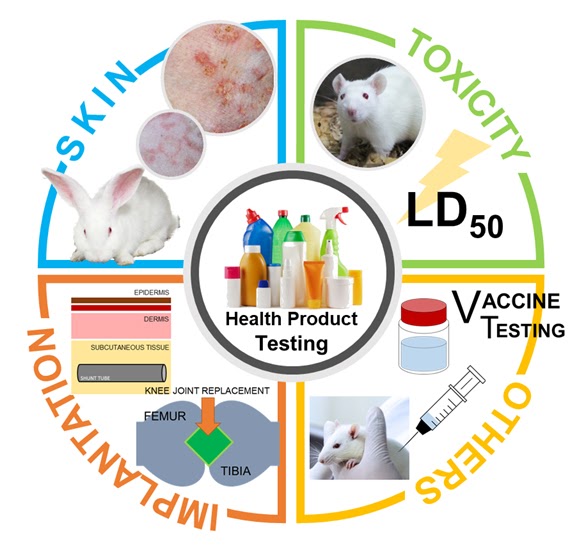
Health and household products are mandatory to be subjected to in vitro or in vivo testing before the products registration by Thailand Food and Drug Administration or FDA. Depending on purposes, each products must be tested using a specific method. For example, skin irritation and/or skin sensitization are occupied with skin care products; oral toxicity are engaged with food and supplement. Hence, the selection of methods are of great importance. NLAC provides various testing methods according to OECD GLP, ISO, NIH, and other international standard guidelines with excellent expertise to recommend the appropriate testing for products. We perform our animal testing in OECD GLP certified environment. With our services, you can ensure that the results are reliable, and competent for the registration by not only national FDA but also by international federation.
By Chanika PINYOROSPATHUM, Ph. D.
